In 2016, the Commission for Hospital Hygiene and Infectious Disease Prevention (KRINKO) at the Robert Koch-Institute (RKI) updated its recommendations for hand hygiene comprising the following specific areas:
The prerequisites for healthcare workers to enter medical working areas are clean hands and fingernails (trimmed short and round; without nail varnish or jewellery). Hygienic hand disinfection is extended to the forearms in case of contamination. When suffering from a chronic skin disease, it is recommended to consult the occupational health physician to eliminate the risk of colonisation with potentially pathogenic germs.

Alcohol-based hand disinfectants are to be kept in the immediate vicinity of the point of care to promote hand hygiene compliance. For this requirement to be met, every bed in intensive care and dialysis wards are to be equipped with at least one disinfectant dispenser, and all other wards should have one dispenser per two patient beds and one in the sanitary area.

Additional portable and fixed disinfectant dispensers on ward and dressing trolleys, in preparation rooms as well as clean and unclean workplaces complete the configuration. Likewise reasonable: pocket bottles that enable staff to disinfect their hands as indicated, regardless of where they are.
When using dispensers equipped with disposable bottles, it is important to avoid microbial contamination of the pump heads. And if not using disposable pumps heads, this means that the dispensers must be reprocessed regularly.
For patient and staff protection, the following five WHO moments require a hygienic hand disinfection with fully wetting all palms and paying special attention to fingertips, nail folds and thumbs:
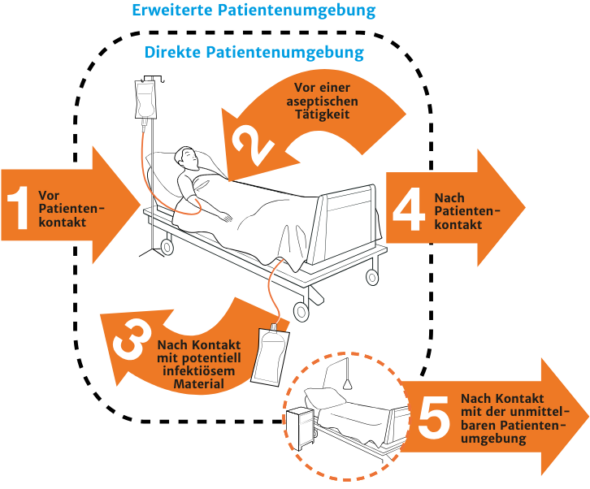
The moments mentioned above apply no matter if single-use gloves are used or not – and: after taking off the gloves, hand disinfection is always required.

Involving patients and visitors in hand hygiene is also a reasonable way to further expand infection prevention.
In case the hands are visibly contaminated, the contamination is removed with a disposable wipe soaked in hand disinfectant, which is then followed by hygienic hand disinfection. If the hands are heavily soiled, they are first carefully rinsed and then disinfected.
The recommendation is to use alcohol-based hand disinfectants listed by the VAH (German Association for Applied Hygiene) and which contain no antimicrobial remanent active ingredients, as they pose a risk of side effects and do not improve the efficacy.
 |
Remanent active ingredients in alcohol-based hand disinfectantsIn addition to containing alcohol, some commonly used hand disinfectants also contain remanent active substances such as chlorhexidine (CHG), mecetronium ethylsulfate (MES) or ortho-phenylphenol (OPP) to improve their sustained activity. However, according to scientific evidence, remanent active ingredients offer no benefit, neither for hygienic nor for surgical hand disinfection – but pose risks to skin health. The current recommendations “Händehygiene in Einrichtungen des Gesundheitswesens” (Hand hygiene in healthcare facilities) and “Prävention postoperativer Wundinfektionen” (Prevention of surgical site infection) issued by the German Commission for Hospital Hygiene and Infection Prevention (KRINKO) therefore advise against using hand disinfectants containing remanent active ingredients. Here you can read a summary of the latest studies. |
The product’s efficacy depends on the pathogen spectrum to be killed, e. g. virucidal against enveloped viruses, limited spectrum of virucidal activity or virucidal. In case bacterial spores, helminths, protozoa and oocysts are present, disposable gloves are put on first – after their removal, hands are first thoroughly washed and then disinfected (hygienic hand disinfection).
As part of quality management, in particular evaluation and feedback measures are to be implemented.

It is also advisable to measure hand disinfectant consumption to feed this data into the HAND-KISS module of the German “Aktion Saubere Hände” (Clean Hands Campaign). New staff members are to be trained in hand hygiene, existing staff members are to be trained at least once a year — direct compliance observations are advisable in case of an increase in nosocomial infections.
Surgical hand disinfection with an alcohol-based hand disinfectant must be carried out before donning sterile surgical gloves and in case of intended direct contact with the operating field, sterile medical devices or before aseptic tasks. The use of a hand disinfectant containing remanent active ingredients is not recommended, as the risks of skin intolerance outweigh the benefits and they do not improve the efficacy.
The prerequisites for entering the operating theatre wing are clean hands and fingernails (trimmed short and round; without nail varnish or jewellery). Hand washing, including the forearms and elbows, is recommended before the first surgery of the day. Enough time between hand cleansing and hand disinfection must be ensured so that any residual moisture on the skin and an associated dilution effect does not impair the hand disinfectant’s efficacy.
If the hands and forearms are dirty, the nails and hands should be cleaned with a soft brush, but not the forearms.
When suffering from a chronic skin disease, the occupational health physician needs to be consulted to eliminate the risk of colonisation with potentially pathogenic germs.

Low-germ gloves are to be put on dry hands to protect against probable or foreseeable contact with secretions, excretions and body fluids. Every glove change requires a hand disinfection – unless in an exceptional case the gloves are disinfected to not interrupt the workflow and in case chemical resistance is fulfilled according to EN 374.
Should the gloves be perforated, visibly contaminated or have been in contact with nonenveloped viruses, they must always be changed. Where protection against chemicals is desired, gloves that are declared as a medical device and as personal protective equipment (PPE) must be used.
Unpowdered sterile surgical gloves with a low latex content must be put on before invasive procedures, when handling sterile medical devices or sterile material. If there is an increased risk of perforation or infection in a patient, the surgical team is advised to either wear two different coloured gloves on top of each other according to the double-gloving method or to change gloves intraoperatively depending on the type and duration of the operation. It is generally necessary to change gloves before accepting an implant in endoprosthetics.
After having finally removed the surgical gloves, hygienic hand disinfection is carried out.

Hands are to be washed before starting work tom remove any bacteria spores and, if necessary, after finishing work to remove any work-related contamination – and always when there is visible contamination. However, it is best to limit hand cleansing to what is absolutely necessary to protect the health of the healthcare worker’s skin.
Also, the reduction of microorganisms achieved by hand washing is inferior to the one achieved by hand disinfection. An exception is the removal of Clostridioides difficile, helminths or protozoa: these can only be removed by washing and not by alcohol-based hand disinfectants.
A skin protection plan has to be established for the health personnel, which includes measures to reduce the risk of wet work and to care for and protect the skin. Nourishing and protective products – preferably without perfume and preservatives – with proven dermatological effectiveness are to be kept available by the employer. Their application is to be trained regularly as part of hand disinfection training.